pDC315,pDC316, pDC311, pDC312, pDC515,pDC516, pBHGlox deltaE1,3Cre, AdMax system-BioVector NTCC质粒载体菌种细胞基因保藏中心
- 价 格:¥17930
- 货 号:pDC315,pDC316, pDC311, pDC312, pDC515,pDC516, pBHGlox deltaE1,3Cre, AdMax system
- 产 地:北京
- BioVector NTCC典型培养物保藏中心
- 联系人:Dr.Xu, Biovector NTCC Inc.
电话:400-800-2947 工作QQ:1843439339 (微信同号)
邮件:Biovector@163.com
手机:18901268599
地址:北京
- 已注册
pDC315,pDC316, pDC311, pDC312, pDC515,pDC516, pBHGlox deltaE1,3Cre, AdMax system plasmids
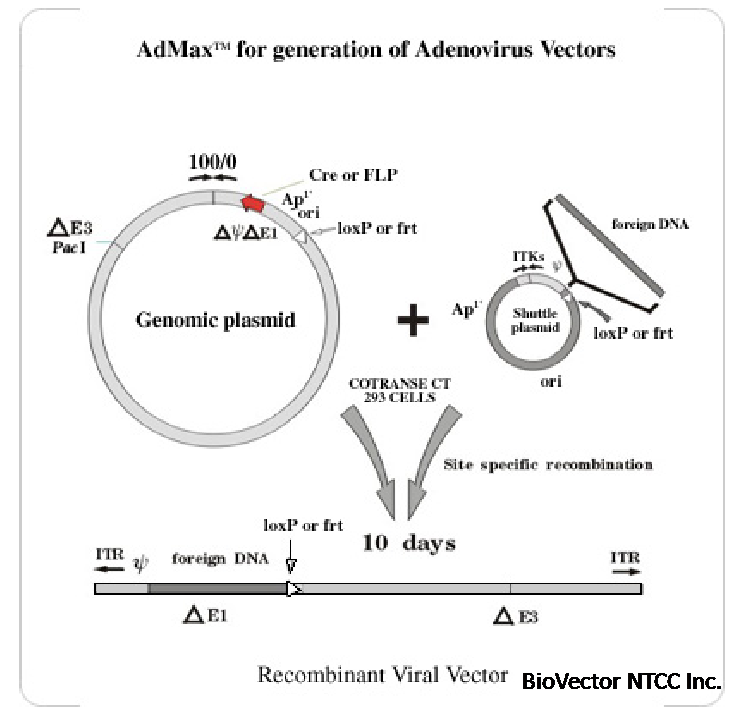
Figure 1 outlines the principles of the AdMax™ system with Cre-lox as an example. Recombination in cotransfected cells
introduces the gene of interest into infectious Ad DNA while simultaneously excising the recombinase gene.
Neither the small shuttle plasmid nor the genomic plasmid need be digested with restriction enzymes prior to cotransfection. Any
E1 complementing cell line such as 293 cells (Graham et al., 1977), 911 cells (Fallaux et al., 1996) or PERC6 cells (Fallaux et
al., 1998) can be used for cotransfections.
Although rescue of viral vectors is highly efficient (over 100 fold greater than with the original two plasmid method of Bett et al.,
(1994)), and 95% of viruses generated by cotransfection should carry the transgene, it is good laboratory practice to build up
working stocks of virus from plaque isolates before extensive experimentation.
Microbix provides low passage 293 cells that are especially cultured to maintain the strong adherence and plaque forming
properties of the original 293 cells. For rapid production of vectors to be used in preliminary experiments, it may be possible to
produce recombinant viruses by incubating cell cultures under liquid medium following cotransfections.
Transgenes are cloned into one of our small high copy number shuttle plasmids (Figures 2 and 4) which are then cotransfected
with an Ad genomic plasmid (Figures 3 and 5) into 293 cells. High efficiency site specific recombination catalyzed by Cre or
FLP recombinase results in "rescue" of the expression cassette into the left end of an E1 deleted (first generation) Ad vector.
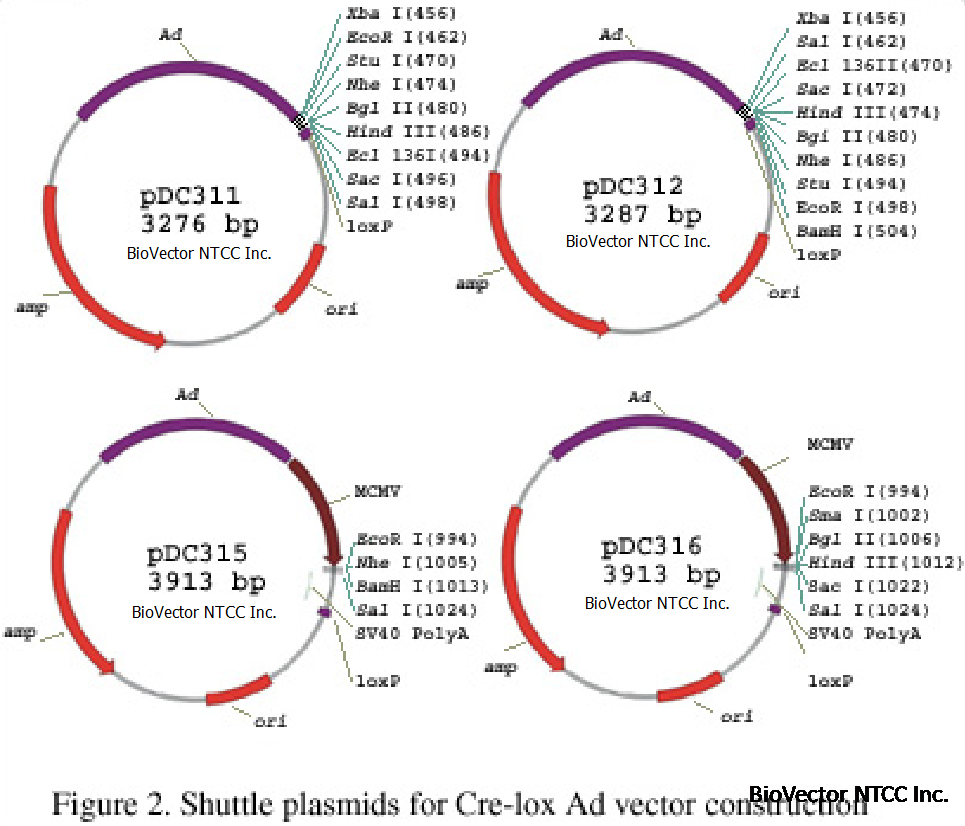
Shuttle plasmids (Figure 2) designed for insertion of the transgene are small, simple and pUC based for high yields.
Promoterless plasmids with polycloning sites comprising recognition sites for 8 enzymes are only 3.2 kb in size. Plasmids
containing an expression cassette utilizing the Murine Cytomegalovirus Immediate Early Gene promoter (MCMV Pr) are only 3.9
kb and have up to 6 restriction enzyme cloning sites. The genomic plasmids containing most of the Ad genome plus cassettes
expressing recombinase and carrying the recombinase recognition site are approximately 34 kb in size. Two recombination
systems are available, based on Cre-lox or FLP-frt.
Figure
图谱maps:
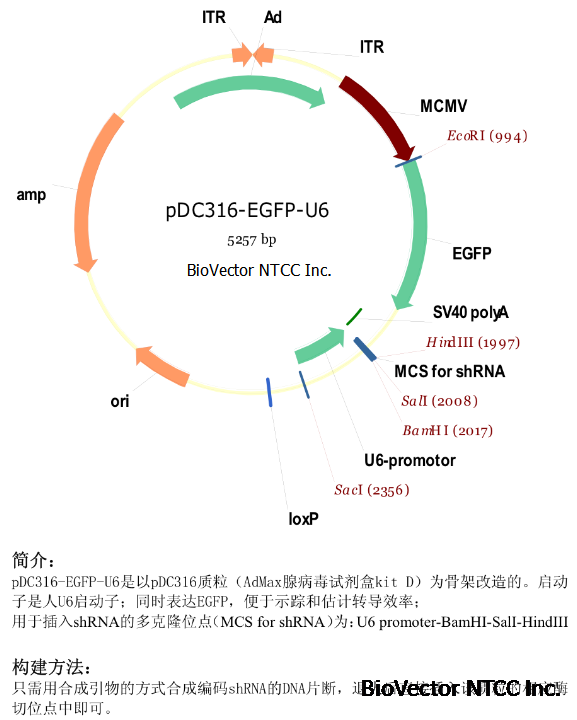
This figure
illustrates a set of shuttle plasmids analogous to those shown in Figure 2 but containing frt sites for recombination by
the site specific recombinase, FLP, encoded by the yeast 2u plasmid (O'Gorman et al. Science 251, 1351, 1991).
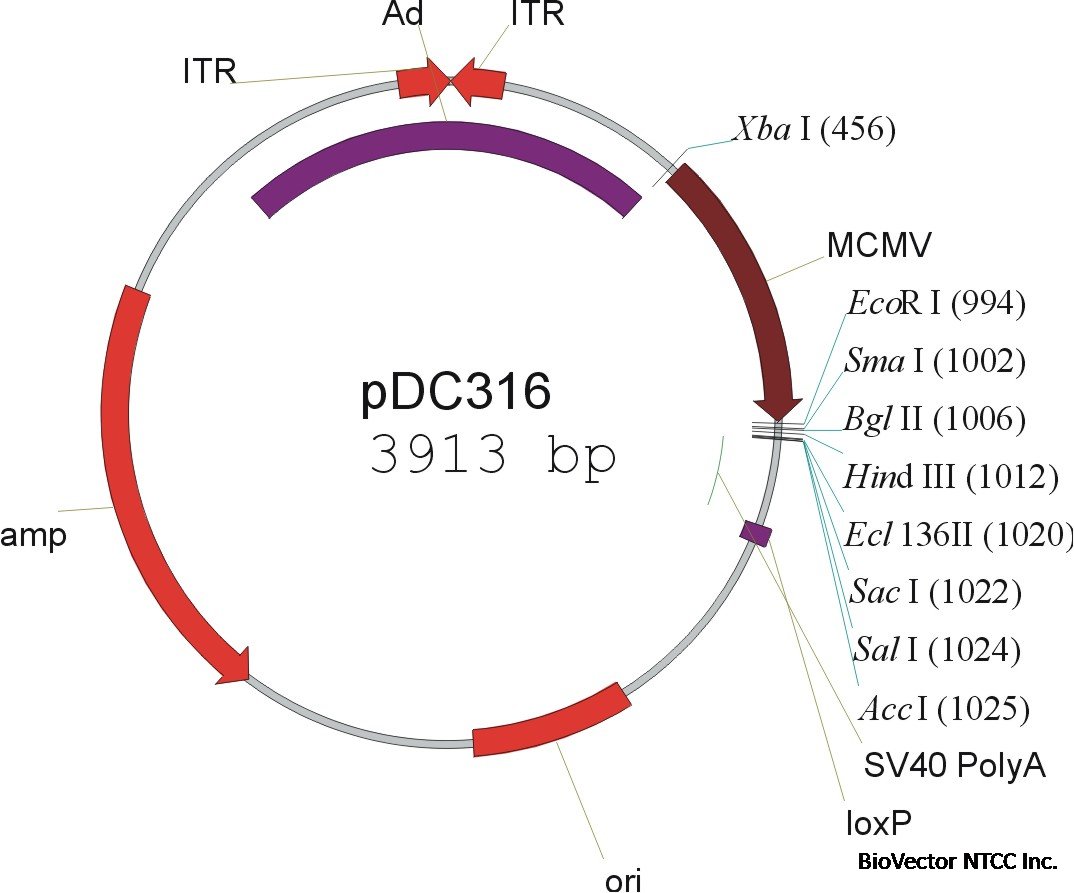
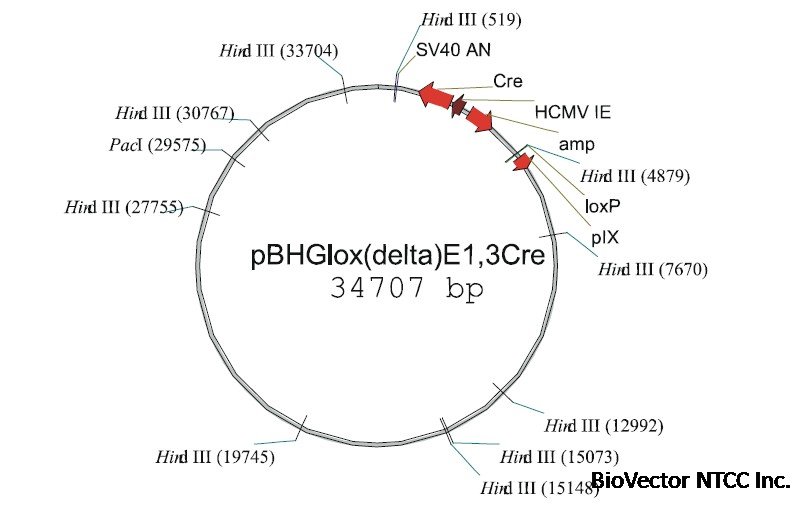
This Figure
shows an example of one of the available Ad genomic plasmids containing a Cre expression cassette (which is
excised during recombination with the shuttle plasmid). This plasmid can be purified and aliquoted and stored frozen for multiple
vector rescue cotransfections. As little as 2 ug DNA/dish suffices to generate numerous plaques following cotransfection of 293
cells with a shuttle plasmid.
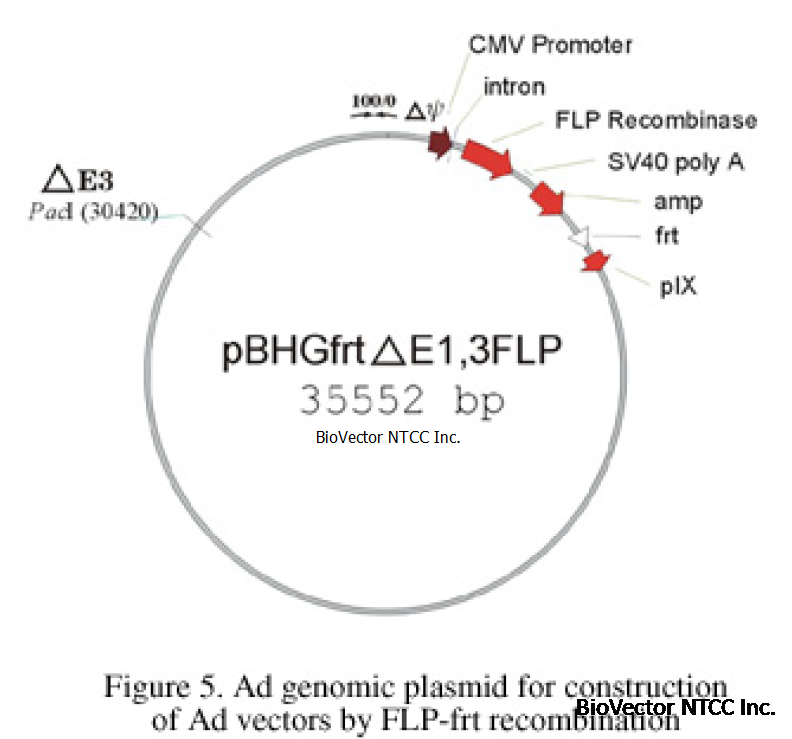

The genomic plasmid encoding FLP and carrying an frt site for FLP mediated recombination with the shuttle plasmids of Figure
4 is illustrated in Figure 5. FLP functions as efficiently as Cre for production of adenovirus recombinants by site specific
recombination between two cotransfected plasmids (Ng., et al., submitted). Plasmids can be propagated in any of the common
bacterial strains such as DH5 alpha.
For recombinant DNA cloning any commonly used protocols will suffice but it is recommended that plasmid DNA to be used in
cotransfections be prepared using the protocol provided with the kits.
Also we recommend that the simple cotransfection protocol provided with the kits be followed as closely as possible at least
initially. Once the users have successfully rescued a number of transgenes and feel comfortable with the system, they are
invited to try other plasmid DNA purification protocols and transfection methods.
For beginners we recommend that initial transfections be done using pFG140 (Graham, 1984), an infectious Ad genomic
plasmid that serves as a positive control and which is provided free with all kits.
Because the only restriction enzymes required with the AdMax™ system are common enzymes used for cloning into the small
shuttle plasmids the AdMax™ system is simpler and more economical than methods requiring rare cutters (Chartier et al., 1996;
He et al., 1998; Mizuguchi & Kay, 1998).
Moreover those rescue protocols typically use enzymes such as Pac I or SwaI to linearize plasmid DNA prior to transfection. If
the transgene contains these sites then these methods are not practical. PacI sites, for example, are found surprisingly often in
eukaryotic DNA. (There is one PacI site in the Murine Cytomegalovirus Immediate Early Gene promoter (one of the strongest
viral promoters available (Addison et al., 1997)) and one also in the gene encoding luciferase, a popular reporter gene.)
The E3 deleted genomic plasmids contain a unique PacI cloning site in E3. It is possible to insert a reporter gene (Parks et al.,
1996) or a gene for a transactivator in the E3 region to create a modified genomic plasmid that can then be combined with
cassettes inserted in the E1 shuttle plasmid. Thus, for example, a series of vectors expressing genes under regulation by tet or
by RU486 can be readily constructed using the AdMax™ system.
Kit D (contains pDC311, pDC312, pDC315, pDC316, pBHGloxDE1,3Cre, and pFG140 )
Kit E (contains Kit E: contains pDC511, pDC512, pDC515 and pDC516, pBHGfrtDE1,3FLP, and
pFG140)
Kit F (contains pDC411, pDC412, pDC415, pDC416, pBHG10, pBHGE3 and pFG140)
References Addison, C. L., Hitt, M., Kunsken, D., and Graham, F. L. (1997). Comparison of the human versus murine
cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J. Gen. Virol. 78, 1653-1661.
Bett, A. J., Haddara, W., Prevec, L. and Graham, F.L An efficient and flexible system for construction of adenovirus vectors with
insertions or deletions in early regions 1 and 3. Proc. Natl. Acad. Sci. US 91: 8802-8806, 1994.
Chartier, C., Degryse, E., Gantzer, M.., Dieterle, A, Pavirani, A., and Mehtali, M. (1996). Efficient generation of recombinant
adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 70, 4805-4810.
Fallaux, F.J., Kranenburg, O., Cramer, S.J., Houweling, A., Van Ormondt, H., Hoeben, R.C., Van der Eb, A.J. Characterization
of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther 7,
215-222, 1996.
Fallaux, F.J., Bout A., van der Velde, I., van den Wollenberg, D.J., Hehir, K.M., Keegan, J., Auger, C., Cramer, S.J., van
Ormondt, H., van der Eb, A.J., Valerio, D., Hoeben, R.C. New helper cells and matched early region 1-deleted adenovirus
vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther 9, 1909-1917,1998.
Graham, F.L., Smiley, J., Russell, W.C., and Nairn, R. Characteristics of a human cell line transformed by DNA from human
adenovirus type 5. J. Gen. Virol. 36, 59-72, 1977.
Graham, F.L., Covalently closed circles of human adenovirus DNA are infectious. The EMBO J. 3, 2917-2922, 1984.
He, T. C., Zhou, S., Da Costa, L. T., Yu, J., Kinzler, K. W., and Vogelstein, B. (1998). A simplified system for generating
recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95, 2509-2514.
Mizuguchi, H., and Kay, M. A. (1998). Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation
method. Hum. Gene Ther. 9, 2577-2583.
Ng, P., Parks, R. J., Cummings, D. T., Evelegh, C. M., Sankar, U., & Graham, F. L. (1999). A high efficiency Cre/loxP based
system for construction of adenoviral vectors. Hum. Gene Ther. 10, 2667-2672.
Ng, P., Parks, R. J., Cummings, D. T., Evelegh, C. M., & Graham, F. L. (1999). An enhanced system for construction of
adenoviral vectors by the two plasmid rescue method. Hum. Gene Ther. 11:693-699, 2000.
Ng, P., Cummings, D. T., Evelegh, C. M. and Graham, F. L. The yeast recombinase FLP functions effectively in human cells for
construction of adenovirus vectors. BioTechniques 29: 524-528, 2000.
- 公告/新闻


 免费订购电话: 400-800-2947
免费订购电话: 400-800-2947